44 legal requirements for dispensing labels uk
4. Veterinary medicines - Professionals ii. for use in GB - in the GB MRL Register as part of the VMD's Product Information Database. d. the veterinary surgeon responsible for prescribing the medicine must specify an appropriate withdrawal period; e. the veterinary surgeon responsible for prescribing the medicine must keep specified records. Antimicrobial and anthelmintic resistance PDF Guidance on Prescribing, Dispensing, Supplying and Administration of ... The prescribing and dispensing/supply and/or administration of medicines should normally remain separate functions performed by separate health care professionals in order to protect patient safety. The joint RCN/RPS document Professional Guidance on the Administration of Medicines in Healthcare Settings(RCN/RPS, 2019)1states that (p3 10):
Dispensing Medicines - PSNC Website Training requirements and resources for COVID-19 vaccinations. PSNC Briefings: National Pharmacy Services. ... The Electronic Prescription Service (EPS) is also being implemented as part of the dispensing service. Service Specification. ... info@psnc.org.uk. Press/Media Queries: commsteam@psnc.org.uk. Telephone: 0203 1220 810. PSNC 14 Hosier ...
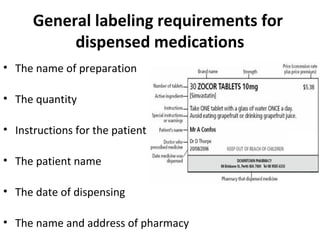
Legal requirements for dispensing labels uk
Prescription writing | Medicines guidance | BNF | NICE The age and the date of birth of the patient should preferably be stated, and it is a legal requirement in the case of prescription-only medicines to state the age for children under 12 years. These recommendations are acceptable for prescription-only medicines. Prescriptions for controlled drugs have additional legal requirements. Dispensing a prescription - PSNC Website Home / Dispensing & Supply / Dispensing a prescription. Print Page. Supply chain and shortages. The dispensing process. Appliances. Medicinal products. Special containers and products requiring reconstitution. Split pack dispensing. Unlicensed specials and imports. PDF Dispensing prescriptions for Controlled Drugs - PSNC Repeat dispensing: Schedule 2 and 3 CDs cannot be prescribed on repeat dispensing prescriptions. Only Schedule 4 and 5 CDs are permitted on repeatable prescriptions. Repeat dispensing prescriptions for Schedule 4 CDs must be dispensed for the first time within 28 days of the appropriate date. After the first dispensing episode is complete, any
Legal requirements for dispensing labels uk. United States Product Labeling Requirements: An Overview - Compliance Gate The Federal Trade Commission (FTC) establishes labeling requirements for importers and manufacturers to adhere to with regard to clothing and textile products. For example, labels on clothing and textiles should be obvious, accessible, and contain information such as the following: Fiber content (e.g. 100% cotton) Optimising Dispensing Labels and Medicines Use The Human Medicines Regulations 2012 introduce changes to labelling and medicines-use which advance the clinical role of pharmacists in supporting people to get the most from prescribed medicines across the UK, providing greater clinical flexibility for prescription intervention. PDF Standard Operating Procedures Dispensing - prescribingadvisor.co.uk with the dispensing software procedures, then go to step 6 2. For manual prescriptions, check if the patient is registered on the practice's dispensing software system. If yes, then add the prescribed items to the patient's record and produce labels in accordance with dispensing software procedures. 3. FDA Issues New RX Label Requirements | RX Label Requirements for Opioids New guidelines from the FDA will ensure that prescription labels are easier to understand and include messaging about the dangers of abuse. On July 1, 2019, the Food and Drug Administration issued new prescription label requirements.. Medication that will be included under these new rules include those that are categorized under the Controlled Substances Act.
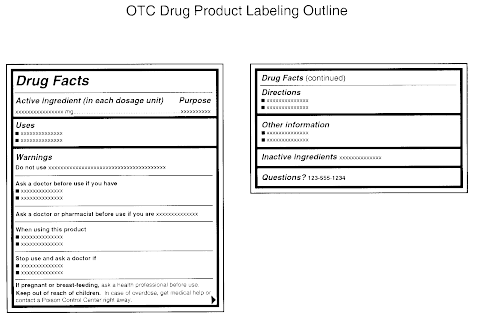
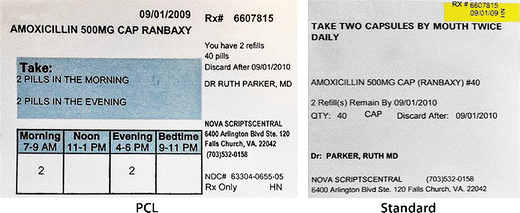
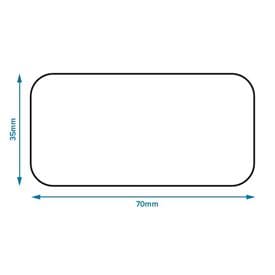
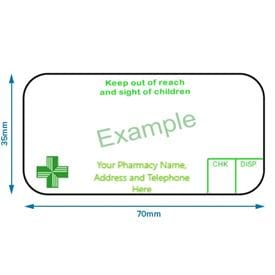
Labelling and packaging - Chemical classification - HSE Under the GB CLP Regulation, there are no significant changes to the labelling and packaging requirements. Hazard labelling for substances and mixture placed on the GB market must be in English... Drug storage and dispensing | BSAVA Library Oral liquids should be dispensed in plain glass bottles with child-resistant closures. All medicines should be labelled. The label should include: The owner's name and address Identification of the animal Date of supply (and, if applicable, the expiry date) Product name (and strength) Total quantity of the product supplied in the container PDF National standard for labelling - Safety and Quality medicines packaging must dedicate a space of at least width 70 mm × height 30 mm for the dispensing label, according to the therapeutic goods administration (tga) therapeutic goods order (tgo) 91.31,32this is a minimum size, and manufacturers are encouraged to follow best practice by leaving as large a space as possible for the dispensed medicine … Ireland - Labeling/Marking Requirements In Ireland, with only minor exceptions, there are no general requirements for marking imported goods with the country of origin. One notable exception is that the Irish authorities require that the name and the EU address of the manufacturer, distributor, or packer also appear on the label. Certain food products must show particulars of place ...
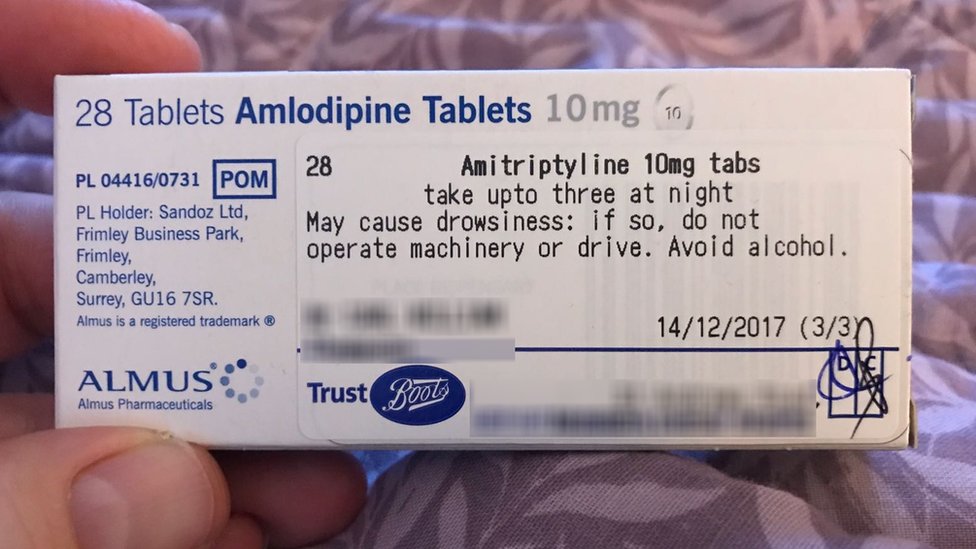
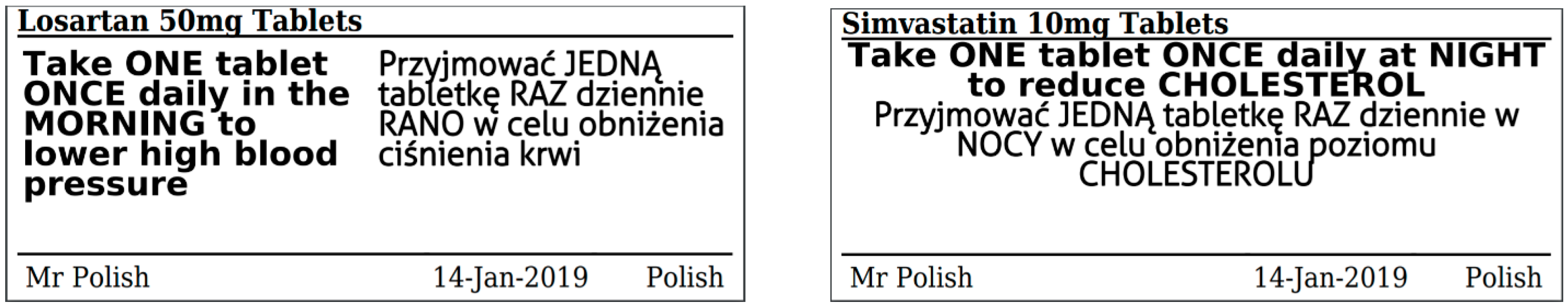
Guidance on Prescribing, Dispensing, Supplying and Administration of ... Guidance on Prescribing, Dispensing, Supplying and Administration of Medicines Some of our publications are also available in hard copy, but this may entail a small charge. For more information and to order a hard copy please call 0345 772 6100 and select option five. The line is open Monday-Friday (excluding bank holidays) between 10am-4pm. Drug storage and dispensing | BSAVA Library Tablets and capsules in foil strips should be sold in their original packaging or in a similar cardboard box for smaller quantities. Preparations for external application should be dispensed in coloured fluted bottles. Oral liquids should be dispensed in plain glass bottles with child-resistant closures. All medicines should be labelled. Labelling standards - Pharmacy Forum UK "apply 1-2 times a day" (bad practice to put numbers on labels also somebody with bad eyesight could see 12) "take two four to six hourly" (quite a few patients probably dont understand this) "take 1 3 times/day" "take ONE cap three times a day (ADVICE) after food" (use proper english!!!!!) "take two morning and night" The Medicines (Labelling) Amendment Regulations 1992 - Legislation.gov.uk Special requirements for the labelling of the name of medicinal products for human use 4D. — (1) In any case where— (a) a relevant medicinal product is available in more than one pharmaceutical...
The Human Medicines Regulations 2012 - Legislation.gov.uk Sale and supply of starting materials. 33. Offence concerning data for advanced therapy medicinal products. 34. Offences: breach of regulations and false information and defence concerning starting materials. 35. Penalties. Conditions for holding a manufacturer's licence. 36.
PDF Dispensing prescriptions for Controlled Drugs - PSNC Repeat dispensing: Schedule 2 and 3 CDs cannot be prescribed on repeat dispensing prescriptions. Only Schedule 4 and 5 CDs are permitted on repeatable prescriptions. Repeat dispensing prescriptions for Schedule 4 CDs must be dispensed for the first time within 28 days of the appropriate date. After the first dispensing episode is complete, any
Dispensing a prescription - PSNC Website Home / Dispensing & Supply / Dispensing a prescription. Print Page. Supply chain and shortages. The dispensing process. Appliances. Medicinal products. Special containers and products requiring reconstitution. Split pack dispensing. Unlicensed specials and imports.
Prescription writing | Medicines guidance | BNF | NICE The age and the date of birth of the patient should preferably be stated, and it is a legal requirement in the case of prescription-only medicines to state the age for children under 12 years. These recommendations are acceptable for prescription-only medicines. Prescriptions for controlled drugs have additional legal requirements.
























Post a Comment for "44 legal requirements for dispensing labels uk"